The Library Network Support for Evidence and Searching (SENSE) services aim to support NHSGGC staff in all aspects of patient care, service development, professional decision making and with their research and CPD. The service can be accessed by all staff via eHelp.
Place a request using eHelp, either by clicking the icon on your desktop or selecting from NHSGGC Favourites in Microsoft Edge.
Select Knowledge Services and the relevant request type.
If you are not on a work computer or device, go to the eHelp login page and login with your network username and password. For support contact ggc.librarynetwork@nhs.scot.
Search services
Literature Search Service
Place a request for a literature search and a professional searcher will search the evidence base and send you a list of relevant articles and other materials, with links to full text if available. If you need support relating to postgraduate and further studies, please request a training session.
Project Support
This is an individually tailored service to support evidence-based projects, such as the development or renewal of clinical guidelines or systematic reviews.
Evidence Summary
An enhanced search and synthesis service to support service improvement, clinical guidelines, patient care and research. It is usually a short and focused piece of work, organising and summarising a list of search results into a single framework or summary. The content of the searches, format of the final results, and timescales are all agreed with the user and tailored where possible to suit the needs of individual projects.
Tools and Measures Enquiry Service
A specialist enquiry service which investigates copyright permissions for diagnostic tools and outcome measures. Where possible, the Library Network will seek to obtain permissions for tools to allow NHSGGC staff to use them. The Library Network does not arrange the purchasing of tools or licences. Please check the Tools and Measures catalogue before you submit a new request. You may need to log in with your usual network username and password to access.
Literature Analysis
A systematic search of the literature, followed by a breakdown and summary of the topics and themes of each reference. This service is available only for large-scale projects to support service development, guidelines and other Board projects.
Literature to Support Strategy and Planning
The Library Network can provide support for strategy, planning and service improvement. Depending on your requirements and timescales, any of the methodologies outlined above can be used. We will usually gather together a team to work on this, and commit a significant resource. In order to help us plan effectively, please engage with us soon as you can.
Support for Systematic Reviews
Systematic review support is based on close collaboration between you and your librarian over a long period of time, for all or part of the lifespan of the systematic review. We require your initial search strategy or research proposal by email before consultation. Please note: this service is not available for reviews carried out as part of postgraduate coursework.
Thanks for your feedback!
The Library Network provides a range of information skills training and support, which you can request via eHelp. Training sessions can take place in person or on Microsoft Teams.
Place a request using eHelp, either by clicking the icon on your desktop or selecting from NHSGGC Favourites in Microsoft Edge.
Select Knowledge Services and the relevant request type.
If you are not on a work computer or device, go to the eHelp login page and login with your network username and password. For support contact ggc.librarynetwork@nhs.scot.
Core Skills
Core Skills training gives NHS Greater Glasgow and Clyde staff the chance to brush up on core writing, reading, number and computing skills used every day. For more information please contact library staff or visit a site library.
Knowledge Skills
Introduction to literature searching
Tailored to your requirements. Introduction to literature searching for relevant articles. Includes forming and planning a search, basic search techniques and locating full text. You will use databases such as Medline; Embase; CINAHL; or PsycInfo.
Advanced literature searching
Tailored to your requirements. For people with previous experience of literature searching.
Finding the fulltext
Accessing full text journal articles via the Knowledge Network, and what to do if the information you want is not available online.
Managing your search results
An introduction to using RefWorks to organise your search results, create a bibliography and create citations in a document.
Finding evidence quickly
An overview of services which help you to access key resources to support clinical decisions at point of care.
Keeping up to date
Suggestions on how to keep up to date with the latest news, research and publications in your area of interest.
Introduction to the NHS Scotland Knowledge Network
Using the Knowledge Network to identify and access relevant information.
Introduction to critical appraisal
Tailored to your requirements. An introduction to how to critically appraise journal articles or guidelines. This training is also available as a group session.
Copyright
To find out more about copyright and the training available please contact library staff or visit a site library.
Survey Tool – Webropol
An overview of Webropol software, which can be used for online surveys, questionnaires and events management. The session is tailored to your requirements.
M365 Skills
The NHS Scotland M365 Skills Hub is now available.
It contains support, advice and training on the M365 resources used in NHS Scotland – Teams, O365/M365, Outlook, Sharepoint, OneDrive, and more.
You should also see a link to the Hub in the left-hand navigation bar in Teams. If you don’t see it, click on the three dots … and click on the M365 Hub app to pin it to your left-hand margin.
eLearning – literature searching
eLearning: A guide to effective literature searching
This module is designed to help the healthcare workforce (clinical and non-clinical) in Scotland build confidence to search published literature for articles and evidence relevant to their work, study and research. It takes 30 minutes to complete and may be ‘dipped into’ for reference or completed to obtain a certificate for CPD. You must sign into Turas first in order to access the content
It is freely accessible to anyone with a Turas Learn account, which is available to all health and care staff in Scotland. If you do not have an account already, please register. This will ensure your learning activity is recorded and you will be able to generate a record of completion.
Contact the Knowledge Network Help Desk with any questions or feedback.
(Originally developed by NHS Health Education England, these modules have been adapted with permission for an NHS Scotland audience by NES Knowledge Services)
Thanks for your feedback!
Do you need help with keeping up to date with new research in your area of interest? The Library Network can help.
Place a request using eHelp, either by clicking the icon on your desktop or selecting from NHSGGC Favourites in Microsoft Edge.
Select Knowledge Services and the relevant request type.
If you are not on a work computer or device, go to the eHelp login page and login with your network username and password. For support contact ggc.librarynetwork@nhs.scot.
Current Awareness Bulletins
Current Awareness Bulletins (CABs) provide details of recent publications in specific subject areas and provide a quick and easy way of keeping up to date.
To see the full list of CABs produced by librarians in NHS Scotland, and to subscribe to receive copies, go to the Knowledge Network current awareness site.
Please let us know if you have any comments about this service, e.g. suggestions for new topics.
Email alerts and RSS feeds
These are used to publish frequently updated information such as new research and publications. Many professional organisations use them to keep readers up to date. Look for links on your favourite web sites and resources and sign up for updates. Library staff are happy to give advice on email alerts and keeping up to date.
Useful links
Here are some useful resources for updates:
Thanks for your feedback!
To find books and articles, go to the Knowledge Network and select the Library Search tab. This catalogue search allows you to:
- Find titles across NHS Scotland’s stock of print books, print journal archives and audio-visual resources
- View and download online journal articles and the full text of national and NHSGGC-subscribed ebooks
- Renew items you have on loan
- Reserve items (whether on loan or not) and have them sent to your preferred library for collection
You need to sign in to view your library record; to renew or reserve items; or to view/download articles and ebooks. On the Library Search page of the Knowledge Network, select Sign In at the top right of the screen. You may sign in here with an NHS Scotland work email address and password if you have one, or an NHS Scotland Athens username and password if you don’t. Once signed in, click the drop-down arrow next to your name to view your library record for loans and renewals.
How to sign in to the Knowledge Network
For information on accessing resources, including how to request items not available from our print and online collections, see the Knowledge Network searching for an item page.
Ensure you sign in via Library Search first before you request a non-stock item. Please note, this service is only available for NHSGGC staff and Partnerships. If you need further help with your request, contact ggc.library.documentdelivery@nhs.scot.
Thanks for your feedback!
The Library Network, NHS Greater Glasgow and Clyde, provides library and information services to all staff working within NHSGGC and its partner organisations.
With nine staffed libraries across NHSGGC, we offer access to an extensive collection of healthcare and associated resources, in print, online and on time.
Library locations
Beatson
The Beatson Library provides specialist cancer information services and resources to all NHSGGC staff and partners.
We are located in the Education Suite, Level 0. Access is available 24/7 using your Beatson swipe card.
Beatson West of Scotland Cancer Centre Library
1053 Great Western Road
Glasgow
G12 0YN
Telephone: 0141 301 7283 (x57283)
Email: ggc.library.beatson@nhs.scot
Gartnavel General Hospital
The Library is in the Administration corridor on the ground floor of the main hospital building, near Medical Illustration’s offices. Library staff are usually available Monday to Friday, 9.00am to 5.00pm. Out of hours access is available for NHSGGC staff: please ask for details.
Library
Gartnavel General Hospital
1053 Great Western Road
Glasgow
G12 0YN
Telephone: 0141 211 3013 (x53013)
Email: ggc.library.ggh@nhs.scot
Glasgow Royal Infirmary
Glasgow Royal Infirmary Library is on the first floor of the New Lister Building. If you enter the building from Alexandra Parade, the library is just behind and to the right of the help desk.
Library staff are available:
Monday: 9.30am-5.00pm
Tuesday to Friday: 9.00am-5.00pm
Please note, this may vary depending on staffing levels.
Out of hours access is available for NHSGGC staff: please ask library staff for details.
Glasgow Royal Infirmary Library
10 Alexandra Parade
Glasgow
G31 2ER
Telephone: 0141 201 5867 (x65867)
Email: ggc.library.gri@nhs.scot
James Bridie Library, New Victoria Hospital
James Bridie Library is on Level 2 of the New Victoria Hospital in the Conference and Education / Staff Facilities corridor. This video provides instructions on how to locate us in the New Victoria Hospital.
24-hour access is available for NHSGGC staff via swipe card.
The library is staffed Wednesday to Thursday from 9.00am to 4.30pm, although this may be affected by staff absences. If you need assistance outside of staffed hours, contact ggc.library.vic@nhs.scot or ggc.librarynetwork@nhs.scot and the library team will be able to assist you.
James Bridie Library
The New Victoria Hospital
Grange Road
Glasgow
G42 9LF
Telephone: 0141 347 8885 (x68885)
Email: ggc.library.vic@nhs.scot
Maria Henderson Library, Gartnavel Royal Hospital
The Maria Henderson Library is the main library for staff working in mental health, psychiatry, psychology, learning disabilities and the community. It is on the ground floor of the Administration Building at Gartnavel Royal Hospital.
The Library is open Monday to Friday, from 9.00am to 5.00pm (closing at 4.30pm on Fridays), and is staffed Tuesday to Thursday, from 9.00am to 5.00pm. The Admin Building front door is locked at 4.30pm, please phone Library staff for access after that time (available on staffed days only). The Admin building is not accessible out of hours; however, nearby Gartnavel General Library has out of hours access. Ask staff for details.
Maria Henderson Library
Admin Building
Gartnavel Royal Hospital
1055 Great Western Road
Glasgow
G12 0XH
Telephone: 0141 211 3913 (x33913)
Email: ggc.library.grh@nhs.scot
Queen Elizabeth University Hospital Campus
The QEUH Library is on the 1st Floor of Queen Elizabeth Teaching and Learning Centre, which is situated between the Institute of Neurology and the Office block.
Access to the library is currently between 8.00am and 6.30pm. Staffed hours are Monday to Thursday, 8.30am to 4.30pm, and Friday, 8.30am to 4.00pm. Please note, between 5.30pm and 6.30pm access to the TLC building is only via the link corridor to the main hospital.
The Library
First Floor
Queen Elizabeth Teaching and Learning Centre
1345 Govan Road
Glasgow
G51 4TF
Telephone: 0141 451 1216 (x81216)
Email: ggc.library.qeuh@nhs.scot
Robert Lamb Library, Inverclyde Royal Hospital
The library is in the Education Centre, within the Inverclyde Royal Hospital campus.
Out of hours access is available 7 days a week. To apply for out of hours access please speak to our team.
Robert Lamb Library
Education Centre (Ground Floor)
Inverclyde Royal Hospital
Larkfield Road
Greenock
PA16 0XN
Telephone: 01475 504402 (x04402)
Email: ggc.library.irh@nhs.scot
Library staffed Monday to Thursday from 9.00am to 4.30pm. Friday 9.00am to 4.00pm.
Royal Alexandra Hospital
The library is situated within the Education corridor at the Royal Alexandra Hospital.
Out of hours access is available on request.
Library
Royal Alexandra Hospital
Corsebar Road
Paisley
PA2 9PN
Telephone: 0141 314 7178 (x07178)
Email: ggc.library.rah@nhs.scot
Library staff available Monday to Friday, from 9.00am to 4.30pm
Stobhill Campus Library
The library is on Level 3 of the New Stobhill Hospital in the Management Offices corridor. You require a Stobhill ACH ID badge for swipe card access. Please contact Security beside Minor Injuries for assistance with access.
Library
The New Stobhill Hospital
133 Balornock Road
Glasgow
G21 3UW
Telephone: 0141 355 1684 (x11684)
Email: ggc.library.stobhill@nhs.scot
Library Staff are usually available Tuesday and Thursday 9.00am to 5.00pm, although this may vary depending on staffing levels.
If you require help when the library is unstaffed, please email ggc.library.stobhill@nhs.scot. Alternatively telephone the library at Glasgow Royal Infirmary on 0141 201 5867 (x65867).
Unstaffed libraries
Vale of Leven
The Library Resource Room at the Vale of Leven is in the Postgraduate Education Centre which is on the 1st Floor of the Community Maternity Building. The Library contains two computers for NHSGGC staff use and a small book collection.
Library Resource Room
Postgraduate Education Centre
Vale of Leven District General Hospital
Main Street
Alexandria
G83 0UA
Access is by keypad. Staff should contact the RAH Library for details.
The Library Resource Room at the Vale of Leven Hospital is part of the Library Services based at the Royal Alexandra Hospital.
Thanks for your feedback!
WE HAVE MOVED HOME! This page is no longer updated, please visit the new Sharepoint Community Nursing Webpage.
To access the New Community Webpage simply CLICK HERE
Hot Topic – Unplanned Catheter Changes
Have you recently read the ‘Urinary Catheterisation for Adults Clinical Guideline’?
This includes some handy troubleshooting guides for UTI’s, Expelled, Blocked and Bypassing catheters.
Best practice states that all patients with an indwelling urinary catheter should carry a Catheter Passport. This is a national document which is a great tool for patients, carers, families and nurses to utilise.
Did you know… If one of your patient’s is seen by OOH’s DN’s for catheter assessment, they will schedule a MIDDAY appointment to the caseload holder in the following days? This MIDDAY appointment should be used to reassess that patients needs.
Formulary – Currently, UnoMedical catheters are the catheter of choice on formulary. Please familiarise yourself with this formulary and aim for 100% concordance. See below for the latest urology formulary.
Please remember to save to your favorites!
Guidance
Urinary Catheterisation for Adults Clinical Guidelines
This is the clinical guideline for Catheter Care for GGC.
NHS Greater Glasgow Urology Formulary
Please use the Urology Formulary to support prescribing for Catheter Care.
NHS Greater Glasgow Urology Formulary
Troubleshooting Support Documents
The documents in this section are to support you when you are troubleshooting patients with Catheters problems. They should be used to support your decision making skills when communicating with patients and their carers via SPOA.
Patient Resources for Catheter Care
The section has resources that you can print and provide to community patients. All patients on District Nursing Caseload should have been supplied with a leaflet as well as verbal instructions and support given.
Cauti
Resources to support patient care with suspected or confirm catheter associated urinary tract infection.
Additional Catheter Care Resources
This section has additional resources to support Catheter Care in Community care.
For guidance for caring for a patient who may experience Autonomic Dysreflexia guidance can be found in the GG&C Urinary Catheterisation for Adults Clinical Guidelines and in appendix 6 from NHS QIS – urinary Catheterisation and Catheter Care.
Thanks for your feedback!
Click on an analyte name below for further information:
Adrenocorticotrophic Hormone (ACTH)
Adrenocorticotrophic hormone (ACTH) is a 39 amino acid peptide hormone secreted by the anterior pituitary, under the control of the hypothalamic peptide, corticotrophin-releasing hormone (CRH). ACTH secretion is pulsatile and exhibits diurnal variation, with highest plasma concentrations around 8am and lowest levels at midnight. It stimulates glucocorticoid (cortisol) production in the adrenal cortex.
ACTH measurement is only useful as a second line investigation following the finding of either cortisol deficiency or excess.
In cortisol deficiency due to primary adrenal failure, ACTH will be raised due to lack of negative feedback. ACTH will be low in adrenal insufficiency secondary to pituitary failure (hypopituitarism).
Excessive production of cortisol accompanied by suppressed ACTH may be seen in Cushing’s syndrome due to adrenal tumours/hyperplasia, and with exogenous glucocorticoid administration.
Cortisol excess with raised ACTH may be seen in ACTH-producing tumours of the pituitary (Cushing’s disease) or other tissues e.g. lungs (ectopic ACTH production).
NB. ACTH secretion may be increased by stress.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: EDTA
- Precautions: Separate and freeze plasma within 4 hours of sample collection. Transport frozen. Timing of collection important. Avoid stress. Haemolysed specimen unsuitable.
- Minimum Volume: 1 mL
- Reference Range: Not applicable
- Turnaround Time: 7 days
- Method: Siemens Immulite
- Quality Assurance: UK NEQAS
Anti-Mullerian Hormone (AMH)
Anti-Mullerian hormone (AMH) is a protein produced by granulosa cells of the ovaries in females and by Sertoli cells of the testes in males.
In women serum AMH concentration increases with age up until the mid-twenties, after which it begins to decline. AMH correlates well with the number of follicles in the ovary (as measured by ultrasound) in women over the age of 25 and its measurement is used to individualise fertility treatment.
In men serum AMH concentration tends to be high in childhood, then declines through puberty to low levels in adulthood. It is used in the investigation of cryptorchidism and anorchidism.
AMH is elevated in the majority of patients with granulosa cell tumours and may be used to monitor disease progression or recurrence. AMH is also useful in the investigation of disorders of sex development as a marker of testicular activity.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: Separate serum and send via first class post. If there will be >48 h before sending store the specimen at -20°C. Sample can be sent at ambient temperature.
- Minimum Volume: 2 mL
- Reference Range:
- Females: <50 pmol/L in young adults (falls steadily towards menopause where it becomes undetectable)
- Males (Levels fall at puberty. These ranges were derived from a study where stage of puberty was not determined):
- 0-1yr 390-1300 pmol/L
- 1-4yr 300-1700 pmol/L
- 5-8yr 260-1200 pmol/L
- 9-12yr 100-1000 pmol/L
- 13-16yr 40-560 pmol/L
- 17-20yr <520 pmol/L
- Adults <100 pmol/L (literature value)
- Turnaround Time: 14 days
- Method: Beckman Access
- Quality Assurance: UK NEQAS
Growth Hormone (GH)
Growth hormone (GH) is a peptide hormone secreted by the anterior pituitary. Its main action is to stimulate the production and release of insulin-like growth factor 1 (IGF-1) by the liver. Excessive secretion causes acromegaly, while deficiency causes failure of growth in children and metabolic problems in adults.
The secretion of GH is very episodic, so random measurement is rarely useful diagnostically.
Failure of GH to suppress during a glucose tolerance test is diagnostic for acromegaly.
Stimulation tests, such as an insulin tolerance test (NB. potentially dangerous, should only be carried out in centres experienced in it) or stimulation with arginine, GHRH/arginine or glucagon, can be carried out to test for insufficiency. GH deficiency may occur as part of a more general deficiency of pituitary hormones, so other hormones are often measured at the same time.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range:
- Random GH:
- > 10 μg/L excludes GH deficiency
- < 0.4 μg/L excludes acromegaly
- Severe Growth Hormone Deficiency:
- Adults Peak GH during ITT < 3 μg/L
- Adults Peak GH with GHRH/Arginine < 5 μg/L
- Children Peak GH during provocation < 5 μg/L
- GH Excess:
- Failure to suppress during OGTT < 1 μg/L
- Mean integrated 24hr GH > 1.7 μg/L
- Turnaround Time: 7 days
- Method: IDS iSYS
- Quality Assurance: UK NEQAS
Insulin-like Growth Factor 1 (IGF-1)
Insulin-like growth factor 1 (IGF-1) is a peptide hormone, very similar to insulin. It is a major growth factor, which is synthesised by most cells and tissues. Circulating IGF-1 is produced by the liver in response to growth hormone (GH). IGF-1 concentration is increased in acromegaly, decreased in growth hormone deficiency and altered in systemic illness and malnutrition.
It is often measured along with growth hormone in the investigation of disorders of GH secretion. It is also used to monitor patients with acromegaly and those on growth hormone therapy.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range:
Age (yr) Males (μg/L) Females (μg/L)
<2 15 – 157 17 – 151
2 – 4 28 – 247 25 – 198
5 – 7 46 – 349 39 – 272
8 – 10 67 – 442 59 – 371
11 – 13 89 – 503 82 – 465
14 – 16 104 – 510 97 – 502
17 – 25 105 – 410 96 – 417
26 – 39 81 – 249 72 – 259
40 – 54 63 – 201 57 – 197
55 – 65 49 – 191 43 – 170
65+ 39 – 186 35 – 168
- Turnaround Time: 7 days
- Method: IDS iSYS
- Quality Assurance: UK NEQAS
Insulin
Insulin, produced by pancreatic beta cells, regulates glucose uptake and utilization and is involved in protein synthesis and triglyceride storage. It is often measured alongside C-peptide.
Clinical uses of insulin measurements:
- Evaluation of possible insulinoma: In cases of hypoglycaemia, diagnosis of insulinoma relies on proving inappropriate secretion of insulin during a hypoglycaemic episode.
- Hypoglycaemia of infancy due to hyperinsulinaemia.
- Diagnosis of factitious hypoglycaemia together with measurement of C-peptide.
- Discrimination of type 1 and type 2 diabetes mellitus: Insulin and C-peptide concentrations are generally low in patients with type 1 diabetes mellitus, and either normal or elevated in early type 2 diabetes, and decreased in later stages.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: Lithium heparin
- Precautions: Collect after overnight fast or during symptomatic hypoglycaemia, together with glucose sample. Separate and freeze plasma within 4 hours of sample collection. Transport frozen. Haemolysed specimens unsuitable. For hypoglycaemic screen, only measure when hypoglycaemic (glucose <2.6 mmol/L).
- Minimum Volume: 2 mL
- Reference Range: Not applicable
- Turnaround Time: 7 days
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
Insulin C-peptide
Insulin C-peptide (connecting peptide), a 31 amino acid polypeptide, represents the midportion of proinsulin. During insulin secretion it is enzymatically cleaved from proinsulin and co-secreted in equimolar proportion with mature insulin. The half life of C-peptide is significantly longer than insulin, so it is detectable in higher concentrations and the level less variable. C-peptide is often a more reliable marker than insulin. In addition, insulin is destroyed by proteases in haemolysed samples, while C-peptide is not.
Clinical uses:
- Insulinoma: Elevated C-peptide levels from increased beta-cell activity.
- Covert self-administration of insulin: Can be virtually ruled out as cause of hyperinsulinaemia by finding elevated C-peptide.
- Type 1 diabetes mellitus: Low C-peptide levels due to diminished insulin secretion, or suppressed as a normal response to exogenous insulin. Patients on insulin can develop anti-insulin antibodies which can interfere with insulin assay, so C-peptide can be used instead to check residual beta-cell activity.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: Lithium heparin
- Precautions: Collect after overnight fast. Separate and freeze plasma. Transport frozen.
- Minimum Volume: 1 mL
- Reference Range: Not applicable
- Turnaround Time: 7 days
- Method: Siemens Immulite
- Quality Assurance: UK NEQAS
Macroprolactin
Prolactin exists in various forms including the monomeric biologically active form (23kDa) and a higher molecular weight form, bound most commonly to IgG, known as macroprolactin (>100kDa). Macroprolactin lacks biological activity but can interfere in standard prolactin immunoassays and is a “common” cause of hyperprolactinaemia (overall prevalence 1.5%). Its presence is determined by recovery of prolactin following precipitation with polyethylene glycol (PEG screening test).
Macroprolactin should be requested in cases of persistently raised prolactin >700 mU/L (on two or more occasions) in euthyroid patients and after excluding drug associated hyperprolactinaemia. PEG screening can identify macroprolactin and determine the concentration of monomeric (bioactive) prolactin, as both may coincide.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range:
- Macroprolactin is reported as positive or negative based on percentage recovery of monomeric (bioactive) prolactin after PEG precipitation to remove macroprolactin:
- Post-PEG recovery <40% – macroprolactin detected
- Post-PEG recovery >60% – macroprolactin negative
- Post-PEG recovery 40 – 60% – equivocal recovery
- Turnaround Time: 7 days
- Method: Polyethylene glycol (PEG) precipitation to precipitate macroprolactin followed by Abbott Alinity immunoanalyser to quantify monomeric prolactin.
- Quality Assurance: UK NEQAS
Parathyroid Hormone (PTH)
Parathyroid hormone (PTH), a polypeptide containing 84 amino acids, is secreted by the chief cells of the parathyroid glands. It has a molecular weight of 9.4 kDa. PTH should be measured in the investigation of unexplained hypercalcaemia or hypocalcaemia. PTH should always be interpreted in light of the serum adjusted calcium concentration and the patient’s renal function.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: EDTA
- Precautions: Avoid haemolysis
- Minimum Volume: 2 mL
- Reference Range: 1.6 – 7.5 pmol/L
- Turnaround Time: 1 day
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
Renin
Renin, a proteolytic enzyme, is synthesized by the juxtaglomerular cells of the kidney and released in response to decreased blood volume, decreased blood pressure and sodium depletion. Renin stimulates aldosterone release through angiotensin intermediates, resulting in the renal retention of sodium and the excretion of potassium.
Renin is measured with paired aldosterone to calculate an aldosterone/renin ratio in the investigation of hypertension.
Renin measurement may be useful in monitoring response to therapy in patients with Addison’s disease or congenital adrenal hyperplasia (CAH).
Beta blockers, diuretics, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, a restricted salt diet and posture can all affect interpretation of renin results.
Sample Requirements and Reference Ranges
- Sample Type: Plasma
- Container: EDTA (Lithium heparin unsuitable)
- Precautions: Do not collect on ice. Separate and freeze plasma within 4 hours of sample collection. Transport frozen. Grossly haemolysed or lipaemic samples unsuitable. Posture and relevant drug therapies (see above) may affect interpretation of results.
- Minimum Volume: 500 μL
- Reference Range:
- Adults (upright): <52 mIU/L
- Infants <1 year: <450 mIU/L
- Children 1 – 5 years: <380 mIU/L
- Children 6 – 15 years: <125 mIU/L
- Turnaround Time: 14 days
- Method: IDS iSYS
- Quality Assurance: UKNEQAS
Sex Hormone Binding Globulin (SHBG)
Sex hormone binding globulin (SHBG) is a large 80-100 kDa glycoprotein that functions to transport sex hormones around the body. It has a high affinity for 17β-hydroxy steroids such as testosterone and oestradiol. Concentrations of SHBG are influenced by many factors. SHBG will be increased by elevated concentrations of circulating oestrogens (including the oral contraceptive pill), hyperthyroidism, liver disease and excess alcohol. SHBG will be decreased by increasing body mass index, polycystic ovarian syndrome and hypothyroidism.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range:
Age Male (nmol/L) Female (nmol/L)
3 – 10 years 45 – 220 50 – 170
10 – 12 years 22 – 188 38 – 129
Adult 13 – 70 20 – 155
- Turnaround Time: 1 day
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
Anti-Thyroperoxidase (TPO) Antibodies
Anti-thyroperoxidase (TPO) antibodies are present in 90-100% of patients with Hashimoto’s thyroiditis, the commonest cause of autoimmune hypothyroidism. Anti-TPO is measured in patients with subclinical hypothyroidism (TSH 5-12 mIU/L and FT4 within reference limits: 8-21 pmol/L) to identify those at increased risk of developing thyroid failure. The risk of developing hypothyroidism if anti-TPO is positive is approximately 5% per year.
Sample Requirements and Reference Ranges
- Sample Type: Serum
- Container: SST
- Precautions: None
- Minimum Volume: 2 mL
- Reference Range: <6 IU/L
- Turnaround Time: 1 day
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
TSH Receptor Antibody (TRAB)
TSH receptor antibody (TRAB) is measured if the cause of thyrotoxicosis is not clear. It is specific for Graves’ autoimmune disease of thyroid but is not present in all cases. It can be used to distinguish Graves’ disease from toxic multinodular goitre and postpartum or subacute thyroiditis. It is also measured in 3rd trimester of pregnancy, if there is a maternal history of Graves’ disease/thyrotoxicosis, to predict risk of neonatal thyroid problems. It may be helpful in cases of possible “euthyroid” Graves’ ophthalmopathy.
Sample Requirements and Reference Ranges
- Sample Type: Serum (plasma unsuitable)
- Container: SST
- Precautions: Grossly lipaemic samples unsuitable
- Minimum Volume: 2 mL (Neonatal samples: minimum serum volume 200 µL)
- Reference Range:
- <3.1 U/L – negative
- ≥3.1 U/L – positive
- Turnaround Time: 14 days
- Method: Abbott Alinity
- Quality Assurance: UK NEQAS
Thanks for your feedback!
Laboratory Sites and Contact Details
Glasgow Royal Infirmary (GRI)
The laboratory is located in the MacEwen Building on Alexandra Parade (adjacent to A and E). It provides routine service Monday to Friday between 9.00am and 5.00pm and on Saturday between 9.00am and 12.00pm. An emergency service operates at all times.
Gartnavel General Hospital (GGH)
The main laboratory is at GGH (in the Laboratory Block of the GGH Complex). This laboratory provides routine and emergency services Monday to Friday between 9.00am and 5.00pm. Between 5.00pm and 7.30pm samples can be sent via the pneumatic tube system, these will be diverted to the porter’s room and sent regularly to GRI for analysis. This service stops at 7.30pm, all samples collected after this time should be sent via taxi to the laboratories at GRI. This taxi should be organised locally at ward level. Any samples sent in the pneumatic tube system after 7.30pm may remain un-analysed until the next day.
New Stobhill Hospital
A small satellite laboratory is located on Level 1 and operates Monday to Friday between 9.00am and 5.00pm.
Enquiries (9.00am until 5.00pm)
There is a central reporting office located at GRI which covers the three North Glasgow laboratory sites.
Duty Biochemist/General Enquiries 0141 242 9500 (x.29500)
Enquiries (Out of Hours)
Contact the on-call biochemist via switchboard 0141 211 4000
Emergency Laboratories (24/7)
Glasgow Royal Infirmary call 0141 211 4487 (x.24487)
Accreditation and Quality
North Glasgow Biochemistry is a medical testing laboratory accredited to ISO 15189:2012 by the United Kingdom Accreditation Service (UKAS). Our UKAS Medical Accreditation number is 9572. A full list of accredited tests can be found on our schedule of accreditation. Tests not on this list are not accredited; please contact the laboratory for further information if required.
The laboratory participates in external quality assurance schemes where available. Performance details are available upon request. If you wish to provide feedback on the North Glasgow Biochemistry service, please contact our Quality Manager.
The Biochemistry department utilises the Telepath Laboratory Information Management System (LIMS) and TrakCare. Due to the limitations of this software, we are currently unable to fully meet the requirements of the UKAS publication GEN-6 – Reference to accreditation and multilateral recognition signatory status.
This publication sets out the requirements of reports/results released by the laboratory to contain the appropriate use of UKAS logos and identify any tests that are accredited and those that are not. The department have risk assessed this. Due to the number of analytes that can be listed on a Biochemistry report, the number of tests that are UKAS accredited and the number of auto comments already added, it is agreed by the laboratory management team that an additional auto comment would detract from the clinically relevant comments and potentially could push these onto a second page where they may be missed altogether. The risk is magnified by the way TrakCare displays results, as any result with a comment has an icon displayed next to it. If an icon is displayed next to almost every result, the alert loses its impact and may lead to clinicians missing critical icons and comments.
Although we are not able to present this information on our reports, the department’s user’s handbook and website provide full details of our accreditation.
Laboratory Handbooks
Forms
During periods of Trakcare downtime, Biochemistry requests must be made on paper request forms. Request forms can be ordered through PECOS, however a pdf copy of the North Glasgow Biochemistry form is available for download and printing.
Laboratory Newsletters
Clinical Audit and User Survey Reports
Thanks for your feedback!
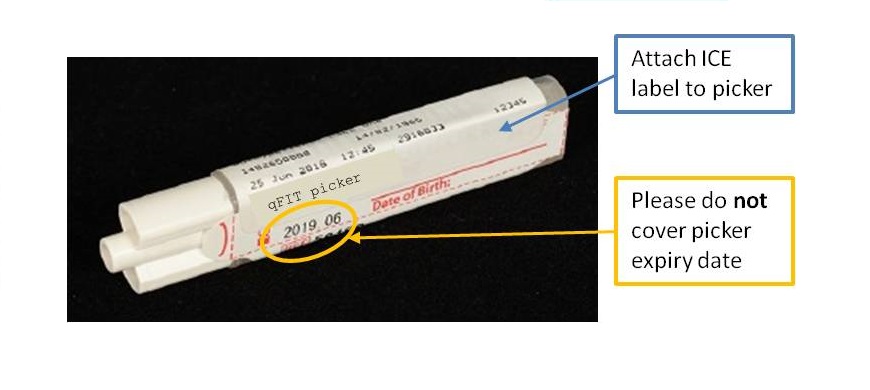
Since Monday 3rd September 2018, quantitative faecal immunochemical testing (qFIT) has been available to Primary Care in NHS Greater Glasgow and Clyde. The service is provided by the Biochemistry department in Glasgow Royal Infirmary. qFIT is designed to detect small amounts of blood in stool samples using antibodies specific to human haemoglobin. Analysis is carried out on the HM-JACKarc system (Alpha Laboratories), with a positive result being 10 µg Hb/g faeces and above.
The NHSGGC Colorectal Cancer Referral Guidance has the latest guidelines and information, along with qFIT patient information leaflets in a number of languages.
Thanks for your feedback!
The Active Staff Programme was set up to Protect and promote health in the workplace by providing a range of free opportunities for all NHSGGC staff to engage in Physical Activity.
We want equitable access by all staff groups regardless of their fitness level, shift pattern or protected characteristics
Engaging in physical activity not only supports good physical health but good mental health also.
Thanks for your feedback!