A new wireless device that could help in the diagnosis of respiratory and sleep conditions in babies and young children is being trialled for the first time with patients.
The Paediatric Advanced Respiratory Service (PARS) has been developed by the West of Scotland Innovation Hub, which is hosted by NHS Greater Glasgow and Clyde, in partnership with digital therapeutics company PneumoWave.
It combines wireless wearable biosensors with mobile app-based software to give real-time analysis of infants’ breathing when they are asleep.
The technology is tailored specifically for children, as many existing in-hospital devices are invasive and therefore poorly tolerated by babies and toddlers.
The sensor is around the size of a 50p coin and only half an inch thick, and is attached using an adhesive pad.
It will also be used to monitor babies in the Royal Hospital for Children’s Neonatal Unit in Glasgow who are at risk of central apnea.
Currently only available for research use only, development of the technology in the PARS project has the potential to improve remote monitoring and diagnostic accuracy in conditions such as sleep disordered breathing, paediatric respiratory disease and acute neonatal compromise.
It is hoped that it will improve patient outcomes and experience, while also adding capacity to clinical services.
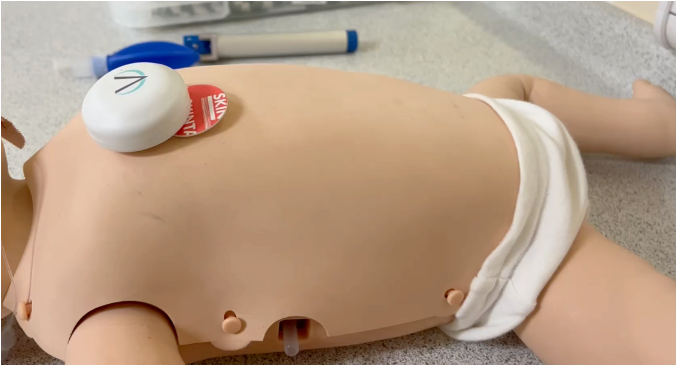
The device and software is now being used in an early stage study with 150 young patients from the Royal Hospital for Children – 75 from the Sleep Clinic Unit and 75 from the Neonatal Unit.
The study, which will run until October 2025, will allow medics to explore how tolerable it is and its feasibility going forward.
Dr Ross Langley, NHSGGC Paediatric Respiratory Consultant, has led the PARS project.
He said: “Detecting and monitoring respiratory problems in children and newborns can be challenging and we know that existing in-hospital devices are invasive and poorly tolerated by children.
“We are pleased that PARS has now entered an early stage study with patients as we explore new ways to diagnose sleep and respiratory conditions in young children.
“This has been developed with industry partner PneumoWave and is tailored specifically for children.
“The device has worldwide potential to improve remote monitoring and diagnostic accuracy in paediatric patients.”
Dr Bruce Henderson, CEO of PneumoWave, said: “Our goal as a company is to stop early deaths from preventable causes, and there is nothing more important than achieving this in children.
“We are privileged to have the support of patients at the Royal Hospital for Children, and of the dedicated staff led by Dr Langley, and grateful to Innovate UK for funding the project.”
The research infrastructure underpinning the study has been supported by Glasgow Children’s Hospital Charity. CEO Kirsten Watson said: “We are delighted that our long-term investment in respiratory research at the children’s hospital has enabled this exciting new development.
“It is crucial that we continue to innovate with minimally invasive technology designed specifically for our most vulnerable babies and children.
“Our heartfelt thanks go to the Reid-Timoney Charitable Foundation for their generous support of our three-year respiratory research strategy.”
It comes as a project designed to develop at-home testing of jaundice in newborns moves a step closer.
The West of Scotland Innovation Hub is working with the North of Scotland Innovation Hub to explore solutions to provide near-patient jaundice testing, as part of a Chief Scientist Office funded Small Business Research Initiative (SBRI).
New innovative testing being developed would use a tiny amount of blood from a heel-prick carried out at home, allowing families to stay together at home rather than babies having to be brought to hospital.
Phase I of the SBRI will see clinicians from NHS Greater Glasgow and Clyde and NHS Highland collaborate with William Oak Diagnostics, who will adapt their micronutrient deficiency Point Of Care Testing technology to detect and measure bilirubin levels in the blood for rapid diagnosis of jaundice.
Jaundice is an almost universal phenomenon in newborns, affecting 60 per cent of babies in the first week of life and current pathways for testing and treatment accounts for six per cent of all admissions to neonatal units.
The challenge originated in 2022 from a proposal submitted by Dr Lesley Jackson and Dr Martina Rodie from the Royal Hospital for Children in Glasgow to the Chief Scientist Office’s Women’s & Children’s Health Catalyst Fund, and has received additional support from Hi: Children’s Healthcare Innovation.
Dr Jackson, co-clinical lead for the project said: “Solving this challenge has the potential to impact on families across Scotland to keep them at home, keep them together, and out of hospital.”
Katriona Brooksbank, Innovation Lead at the West of Scotland Innovation Hub, said: “Collaboration with colleagues from across the country illustrates how we can best use resources to develop innovative solutions that enhance patient care.
“The use of both urban and rural testbeds will allow any processes or systems developed to be tested in different ways, which will contribute to how we make any developments fit for purpose.”
Hazel Dempsey, Innovation Programme Lead from the North of Scotland Innovation Hub, said: “We are really excited to be collaborating with our colleagues in the West of Scotland Innovation Hub to deliver this innovative project.
“Co-designing and testing in a large urban centre provided by NHS Greater Glasgow and Clyde, and remote and rural settings provided by NHS Highland helps ensure that solutions developed can be applicable across Scotland.”
